Concrete is the most widely used man-made material today on earth, with portland cement being the most common type of binder used in its production. Cement-based
materials gain their strength and other properties through the process of hydration, which includes chemical reactions between cement components and water.
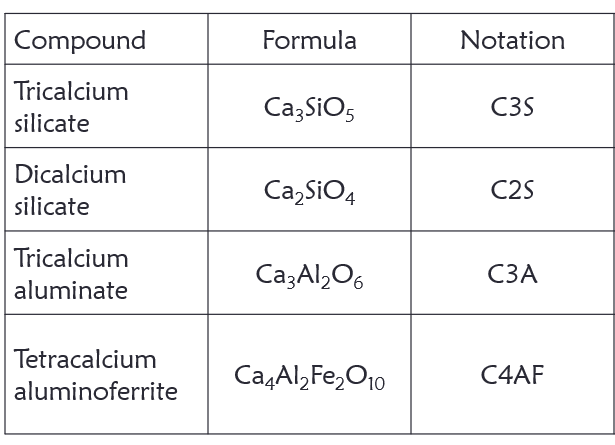
Portland cement is made from various raw materials that act as a sources of primarily lime, silica, alumina and iron oxide. During the production process, the raw materials interact to produce a more complex compounds namely; tricalcium silicate,
dicalcium silicate, tricalcium aluminate, and tetracalcium aluminoferrite.
Cement Compounds
- C3A
It generates a lot of heat during the early stages of hydration, but contributes little to strength. Addition of gypsum to cement during the manufacturing slows down the hydration rate of C3A. Cement low in C3A is sulfate resistant
- C3S
This compound hydrates and hardens rapidly. It is largely responsible for portland cement’s initial set and early strength gain. around 70% of C3S reacts by 28 days
- C2S
C2S hydrates and hardens slowly. It is largely responsible for strength gain after 28 days
- C4AF
This is a fluxing agent which reduces the melting temperature of the raw materials in the kiln. It hydrates rapidly, but does not contribute much to strength of the cement paste.
—————————————————————————————————————————————–
According to ASTM C150, portland cement is classified into different types according to their chemical compositions and particle sizes (or specific surface areas):
- Type I Normal cement
- Type IA Normal + air entrained agents
- Type II Moderate sulphate resistant cement
- Type IIA Moderate sulphate resistant cement + air entrained agents
- Type III High early strength cement
- Type IIIA High early strength cement + air entrained agents
- Type IV Low heat cement
- Type V High sulphate resistant cement